
A new approach to culturing a critical component of tumours, integrating advanced cellular analysis techniques with biomaterials engineering, offers promising avenues for developing more effective cancer treatments and improving patient outcomes. The process, developed by a team of researchers led by Assistant Professor Eliza Fong (Biomedical Engineering), makes use of a bioengineered hydrogel to help clinicians better understand the diversity of cells within tumours and how they influence cancer progression.
The team focused in particular on a population of cells called cancer-associated fibroblasts (CAFs), which come in a variety of different subsets or states. Each of these CAFs has unique effects on how cancer grows and different responses to treatment.
The research was undertaken in collaboration with the National Cancer Centre Singapore and the team’s findings were published recently in the journal Advanced Science.
“Alongside cancer cells themselves, these fibroblasts play a vital role in tumour growth and drug response,” said Asst Prof Fong. “However, until now a lack of suitable models to study these diverse fibroblast types outside of the body has been a major challenge.”
To address this, Asst Prof Fong and her team made use of a customisable hydrogel made from hyaluronic acid, a substance commonly used as a dermal filler in cosmetic dermatology and which occurs naturally in connective tissues, skin, and eyes. This gel was designed to mimic the conditions inside the body and was found to support the culture of different fibroblast types.
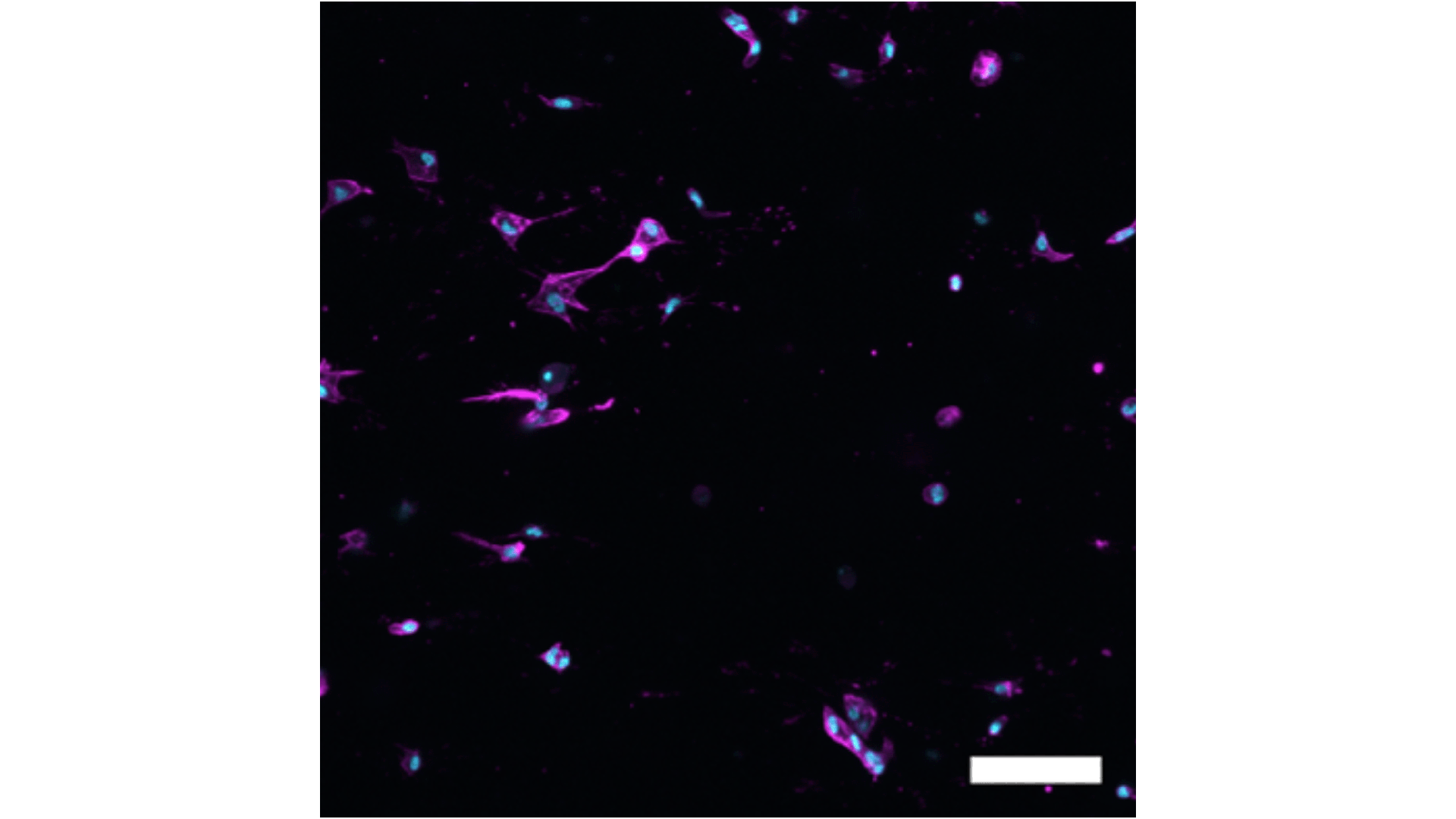
By analysing data from individual head and neck cancer patients, the researchers were able to tailor the hydrogel to simulate the specific conditions that influence the behaviour of different fibroblast types.
“What’s unique about our approach is the integration of single-cell RNA sequencing analysis with biomaterials engineering,” said Asst Prof Fong. “For our research, we leveraged patient single-cell RNA sequencing data sets, extracted information about what could possibly govern these different fibroblast states, and then reflected these microenvironmental cues into the hydrogel.”
Whilst the researchers say more work is needed before this can be applied in clinical settings, they say their breakthrough has significant implications for future cancer drug development.
“Pharmaceutical companies are already showing interest in using these new models to study how different fibroblast types respond to drugs and contribute to cancer progression,” said Asst Prof Fong. “In our study we found that different fibroblast types responded differently to standard-of-care drugs used for head and neck cancer, a finding that could revolutionise treatment approaches.”
Looking ahead, the researchers plan to further refine their models by incorporating different fibroblast types with microscopic, lab-grown models of tumours, called cancer organoids, and immune cells. This, they say, will enable more accurate testing of new cancer treatments, particularly immunotherapies.
The team behind this research included researchers from the Translational Tumour Engineering Laboratory at the Department of Biomedical Engineering, The N.1 Institute for Health at NUS, National Cancer Centre Singapore and Duke-NUS Medical School.





