Into the world of gut microbes
Follow CDE
PDF Download
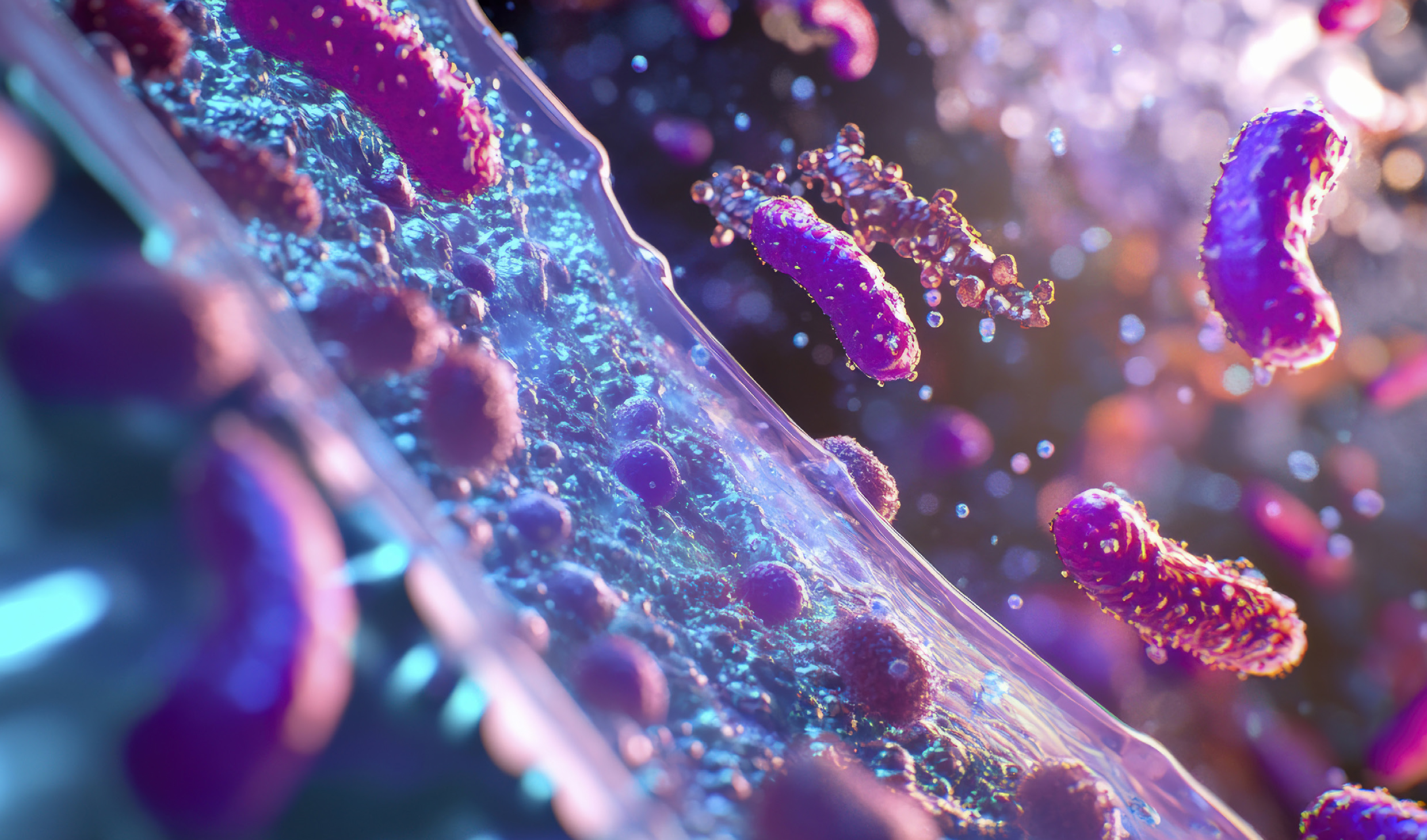
A scalable, reproducible “micro-gut” model opens up new possibilities for studying the links between gut microbes and human diseases, paving the way for improved treatments and therapies.
There’s a whole other world inside of us. One that a constellation of microbes call home. Collectively, these microscopic critters — about 39-trillion-strong — outnumber our own cells.
Residing mainly in the gut, these tiny microbes play an outsized role. Beyond facilitating the breakdown of food, they also have a hand in many vital physiological processes of the human body, from regulating blood sugar to boosting the immune system to modulating mood, stress responses and even food cravings.
Given its importance, it is unsurprising that when this ecosystem of microbes is thrown out of kilter, our health suffers. Unhappy microbes can wreak havoc on gastrointestinal tissues, leading to disorders such as inflammatory bowel disease. They can also accelerate unhealthy ageing, increasing the likelihood of conditions such as obesity, cancer and Alzheimer’s. Promisingly, bringing these connections to light means new types of treatments could be developed. But first, the underlying mechanisms behind these connections need to be understood.
Professor Lim Chwee Teck led a team to develop a novel Gut Microbiome-on-a-Chip that reveals how gut microbes interact with each other and the body.
And what better way to dissect the gut microbiome than in its natural habitat? But how can that be done when the gut is tucked away inside the body? Professor Lim Chwee Teck has a solution: a miniaturised physical twin of the organ.
At the Department of Biomedical Engineering, College of Design and Engineering, National University of Singapore (NUS), Prof Lim, who also serves as the Director of the NUS Institute for Health Innovation and Technology (iHealthtech), led a team to develop a novel Gut Microbiome-on-a-Chip (GMoC). The chip replicates the complex environment of the human gut, providing a scalable, reproducible in vitro model for studying how gut microbes interact with each other and the body. This will help researchers better understand the mechanisms behind microbe-induced diseases and identify new therapeutic targets — all of which could lead to more effective treatments and better health outcomes.
The team’s findings were published in the journal Advanced Science on 27 February 2024.
A window into the life of gut microbes
From bacteria to viruses to fungi, communities of microorganisms — collectively known as the gut microbiome — live in our intestines as the unsung heroes of gut health. Yet, when a spanner is thrown into their works, they can also become contributors to disease.
Deciphering the precise ways in which these microbes either protect or harm the gut is key to developing targeted, preventative healthcare. However, current methods to study the microbiome, such as animal models and static in vitro cultures, struggle to capture the dynamic environment of the human gut. These methods also lack the capability for high-resolution, real-time imaging, making it challenging to observe the detailed interactions between microbes and their impact on gut health.
Prof Lim’s solution came in the form of a microfluidic chip no larger than a coin. But make no mistake — it’s a powerhouse of innovation. The GMoC system features a 3D version of the gut epithelium that recreates key structural and functional aspects of the intestinal tract — from the nutrient-absorbing intestinal villi to the co-existence of microbes and intestinal cells under the dynamic conditions of food movement.
“Replicating the structure of the intestinal villi is important because where various microbial species reside influences how they behave and function,” adds Prof Lim. “The system also produces mucin, which acts as a line of defence against microbial invasion and forms the basis of the gut-bacteria interface.”
"Replicating the structure of the intestinal villi is important because where various microbial species reside influences how they behave and function."
"Replicating the structure of the intestinal villi is important because where various microbial species reside influences how they behave and function."
In addition, the micro-gut incorporates high-resolution imaging, offering researchers an unprecedented view of microbial behaviours in real time. This provides an unobstructed window into how different microbes compete, cooperate and control gut health. For instance, it can reveal how beneficial bacteria in probiotics, or specific nutrients and drugs, interact with resident microbes to influence gut health. As the gut microbiome tends to lose diversity with age, these insights could help devise strategies to restore a healthy, diverse microbiome and promote healthier ageing. Visualising gut microbes can also help decode how they interact with gut tissues, shedding light on how harmful microbes trigger disease and how beneficial ones protect and modulate vital pathways.
"Replicating the structure of the intestinal villi is important because where various microbial species reside influences how they behave and function."
To see this complex interaction in action, the researchers introduced both harmful Enterotoxigenic Bacteroides fragilis and beneficial Lactobacillus species into the GMoC. Interestingly, they found that while the harmful bacteria damaged the gut lining and triggered signals that could lead to tumour growth, introducing Lactobacillus beforehand effectively curbed these effects by preventing the harmful bacteria from taking hold, thus preserving gut health.
More than a gut feeling
“The GMoC system represents a step change in our ability to investigate the effect of the gut microbial community on gut health and diseases,” says Prof Lim. “By establishing a physiologically relevant gut model capable of culturing communities of gut microbes, we can gain deeper insights into the role and complex mechanisms of these microorganisms in maintaining gut health and preventing disease.”
Looking ahead, Prof Lim’s team aims to refine the GMoC system further, making it even more representative of the human intestine. To do so, they plan to enhance its sophistication — from integrating mechanical cues to increasing cellular diversity to creating oxygen gradients within the system.
The researchers will also use the platform to explore how microbial communities form, interact and respond to various stimuli, including nutrients and antibiotics. Commercialisation is also on the team’s radar. “We are currently in talks with companies interested in using the technology for applications like drug screening and nutrition studies,” adds Prof Lim.
Read More
View Our Publications ▏Back to Forging New Frontiers - October 2024 Issue
If you are interested to connect with us, email us at cdenews@nus.edu.sg