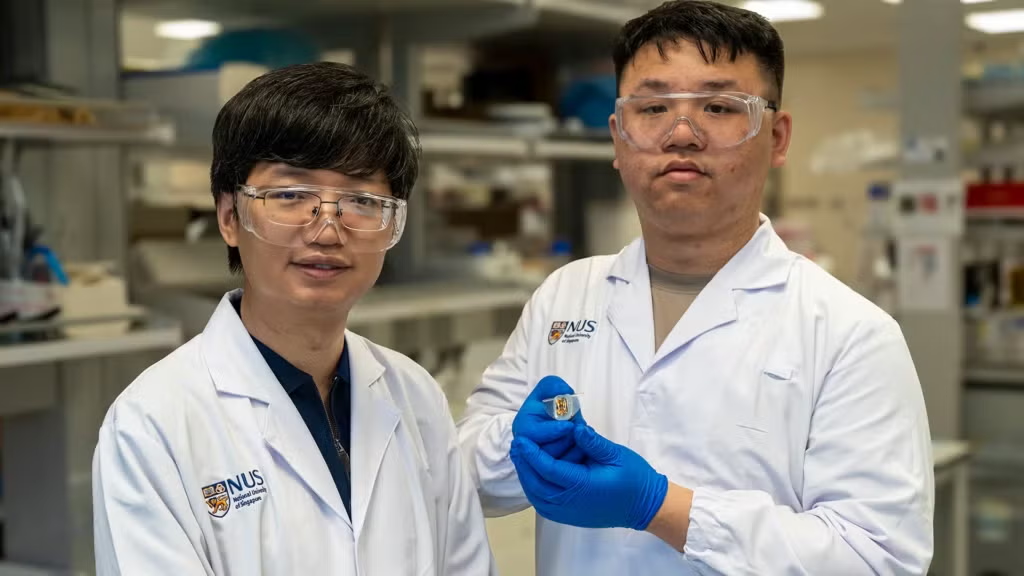
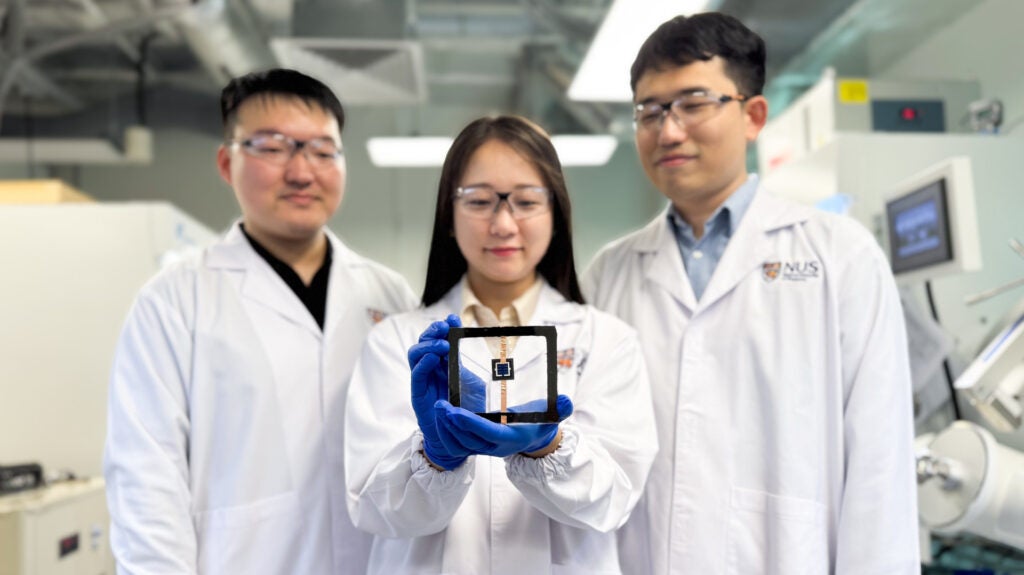
A new class of intelligent material that could be used in a range of applications from drug delivery to energy storage has been developed by NUS researchers, led by Professor Antonio Castro Neto, (Dept of Materials Science & Engineering).
The intelligent material dubbed “2D-electrolytes” is just one atom thick and as with other intelligent materials alters its shape in response to changes in its environment.
Like traditional electrolytes, these new “2D-electrolytes” dissociate their atoms in different solvents, and become electrically charged. Furthermore, the arrangement of these materials can be controlled by external factors, such as pH and temperature.
The breakthrough material was developed by a team of researchers at the NUS Centre for Advanced 2D Materials (CA2DM) led by Prof Castro Neto, who is also faculty at the NUS Department of Physics.
Cancer treatment
One area in which the researchers say the new material could prove especially useful is in healthcare, where it could be used to direct drugs to a specific target inside the body.
This is particularly important for treating diseases like cancer, as the material only releases the drug payload when it detects the presence of a cancer cell, leaving healthy cells unharmed.
The 2D-electrolytes also show promise for other applications that require a material to be responsive to environmental changes, such as artificial muscles and energy storage.
Recent years have seen an increasing number of 2D materials developed. These are solid materials that exists in a single layer of atoms. Having a specific height and width, but effectively no depth, they are essentially two-dimensional.
An electrolyte, meanwhile, is a substance that produces an electrically conducting suspension when dissolved in a solvent, such as water.
This behaviour has been well-established in many other compounds, but the material developed by NUS researchers is the first to have both 2D structure combined with the properties of electrolytes, with a particular trend to shapeshift their form reversibly in a liquid medium.
For more on this research, click here to visit NUS News
* Article from NUS Engineering news