-
-
- Bridging Scales from Below: The Role of Heterogeneities in the Global Water and Carbon Budgets
- Increasing Occurrences of Cyanobacterial Blooms Driven by Climate Change Factors
- Carbon Capture and Utilization
- Integrated Coastal-Inland Flood Model for Climate Change
- Pathways for Sustainable and Climate-Resilient Planning of Water-Energy-Food Security Nexus
-
- Air Quality and Health: A Paradigm Shift
- Surface Water Quality and Emerging Contaminants
- Microbial detoxification of persistent organohalide pollutants (POPs)
- Nutrients Removal in Waterbodies via Sustainable Pathways
- Centre for Water Research (CWR) researchers join their forces with U of T researchers for microplastics pollution detection and control in water and wastewater
- Dealing with Hard-To-Treat Industrial Wastewater
- Valorization of Bioresources – Towards a Circular Economy
-
- Intelligent Traffic Diffusion Plan Generation, Effective Assessment and Dissemination Strategies
- Transforming Waste into Resources for Infrastructural Development
- Look-Ahead Integrated Geophysical Investigation System (IGIS) for Singapore Tunnels
- Next-Generation Airport Pavements with Full-Scale Instrumented Testing
-
- Centre for Advanced Materials and Structures (CAMS)
- Centre for Hazards Research (CHR)
- Centre for Resilient Underground Infrastructure and Engineering (CRUISE)
- Centre for Transportation Research (CTR)
- Centre for Water Research (CWR)
- Centre for Resource Circularity and Resilience (CR)2
- Centre for Offshore Research and Engineering (CORE)
- Centre for Environmental Resilience (CES)
- Safety & Health Committee
- Completed Research Projects
- Research Brief
- Achievements (in the media)
Valorization of Bioresources – Towards a Circular Economy
Biomass waste, such as food waste, garden waste, paper waste, etc., contributes to approximately 40% of the total waste generation in Singapore. According to the Zero Waste Masterplan, changing from the “take-make-dispose” linear economy to circular economy is considered important to reducing waste disposal and cutting carbon emissions. Such a transition needs to be actualized with cutting-edge technologies in place for closing the bioresources loop. Our research strives to valorize bioresources with innovative engineering solutions. In particular, we are interested in green and sustainable technologies for the upcycling of biomass into bio-based chemicals and materials, such as platform molecules, fuel additives, bio-based polymers, which are renewable alternatives to their petroleum-derived counterparts.
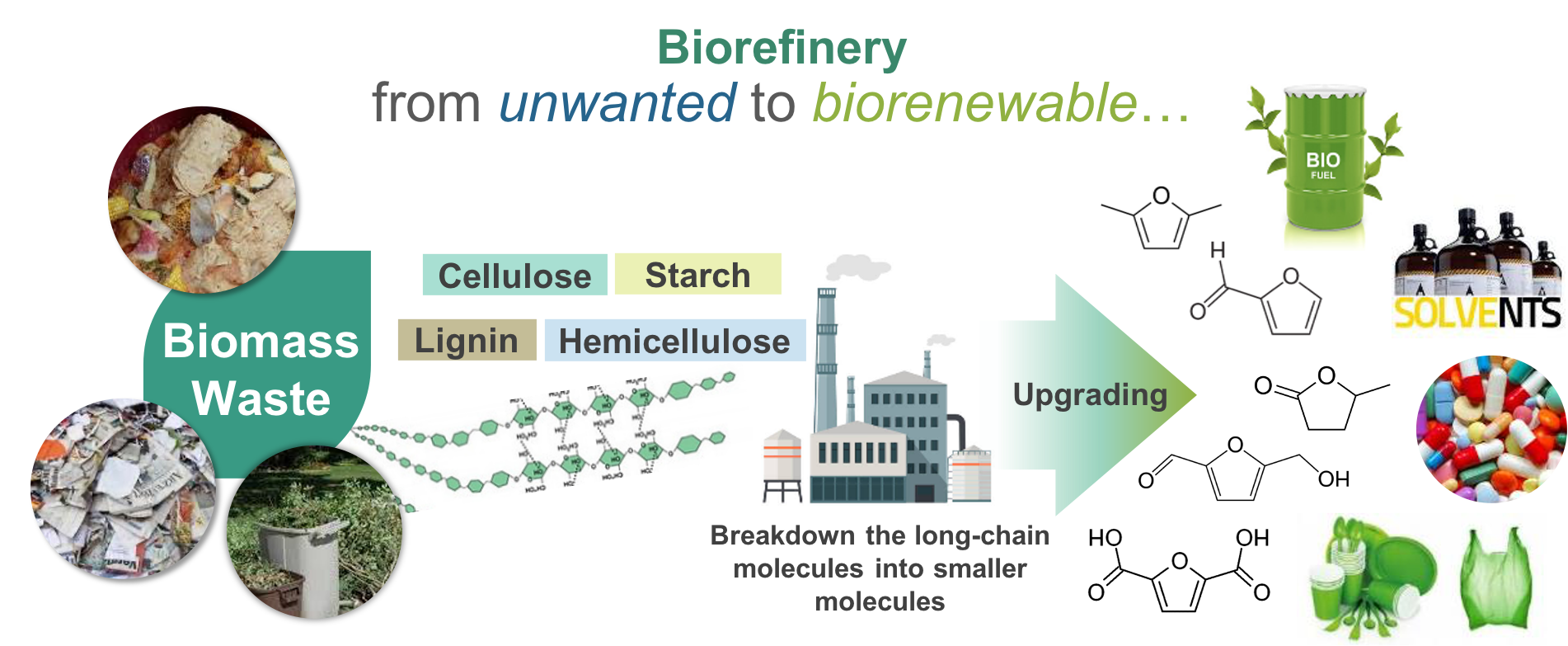
Figure 1. Concept map of bioresource valorization.
One of our projects focuses on the use of microwave technology for energy-efficient thermochemical processing of biomass (Fig. 2a). Our study reveals strong evidence of interactions between microwave and starch that induce superheating for glycosidic bond cleavage (Fig. 2b-d). We speculate that the functional groups of starch can serve as microwave radiators at elevated temperatures, whereas the bound water molecules in the gelated starch matrix undertakes dipolar polarization generating frictional heat. As a result of self-improved dielectric heating, the microwave system achieved an energy reduction by 47% compared to conventional autoclaving. The addition of common, non-toxic salts such as NaCl can further reduce the energy consumption by 70%, as the salt enables superior dielectric heating via ionic loss under microwave irradiation (Fig. 2e). It is noteworthy that the product yields from microwave-assisted hydrolysis of starch were similar to that under conventional heating when reactors with similar configurations were used in comparative study (Fig. 2f). Our better understanding of microwave activation is essential for rational engineering of microwave-assisted processes for biorefinery with maximal efficiency.
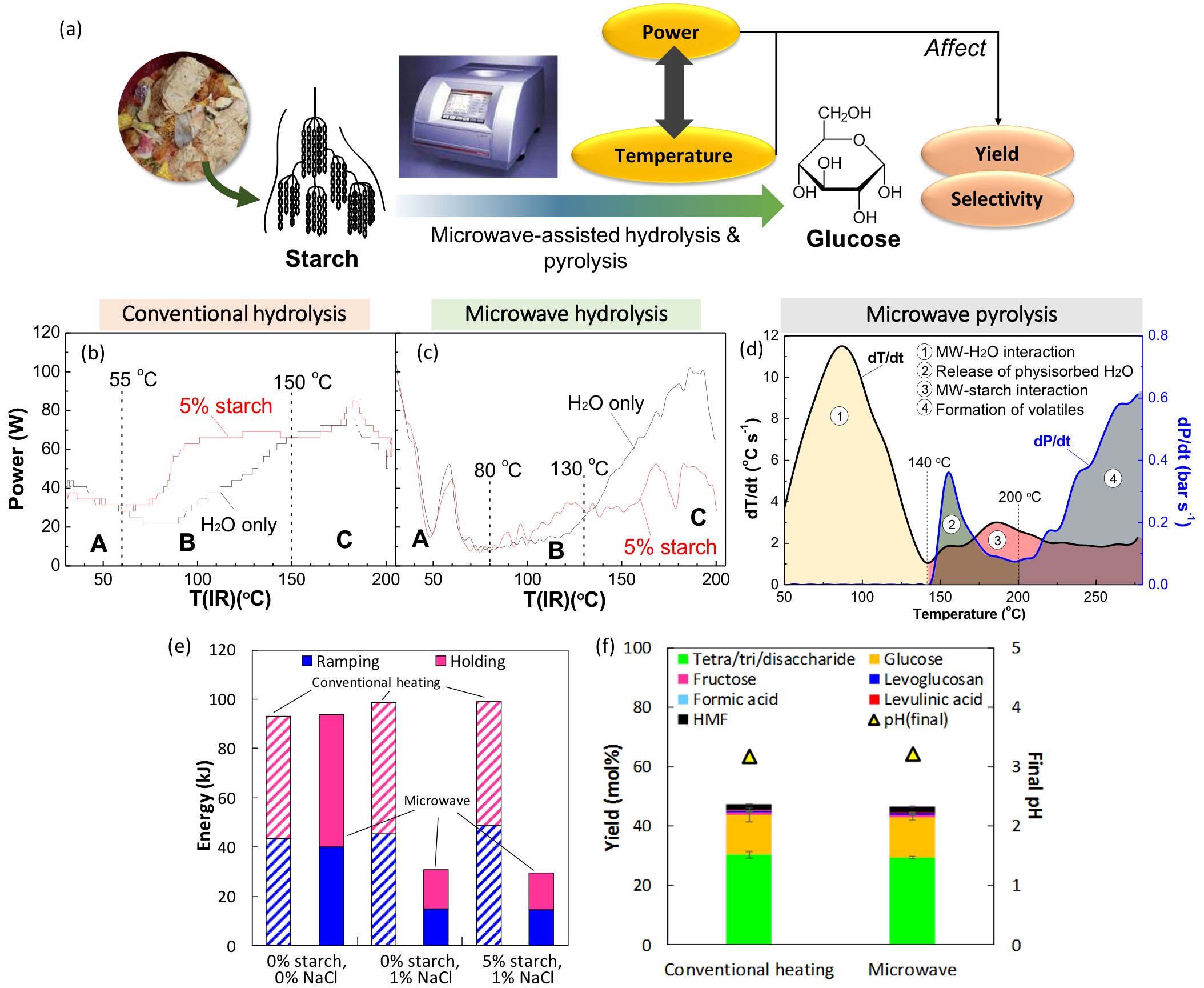
Figure 2. (a) Microwave-assisted conversion of starch as a food waste component; comparison of temperature profiles between (b) conventional heating and (c) microwave heating for hydrolysis of starch; (d) temperature profile of microwave pyrolysis of starch; (e) energy consumption of different heating systems; (f) product yields from hydrolysis of starch under conventional and microwave heating in the presence of 1 wt% NaCl. (Green Chem., 2020, 22, 7109-7118; Green Chem., 2020, 22, 7355-7365)
Catalytic upgrading of bio-based chemicals is one of vital stages in the bioresources loop. For instance, the catalytic hydrogenation of bio-derived furanic compounds would generate renewable fuel additives (e.g., dimethylfuran) (Fig. 3a). Our recent project investigated the solvent impact on Pd-catalyzed hydrogenation of furfural in aqueous phase, shedding light on the fundamentals of the bimolecular reaction through the study of kinetic isotope effect, adsorption thermodynamics, and kinetic model of hydrogenation. It was evident that the hydrogenation rate increased with the decreasing solution pH (Fig. 3b). The higher concentrations of hydronium ions weaken the binding of hydrogen on Pd surface, which favors the transfer of the hydrogen to furfural (Fig. 3c). The way the solvent controls kinetics and thermodynamics by impacting the co-adsorbates inspires a new perspective to engineer catalytic systems.
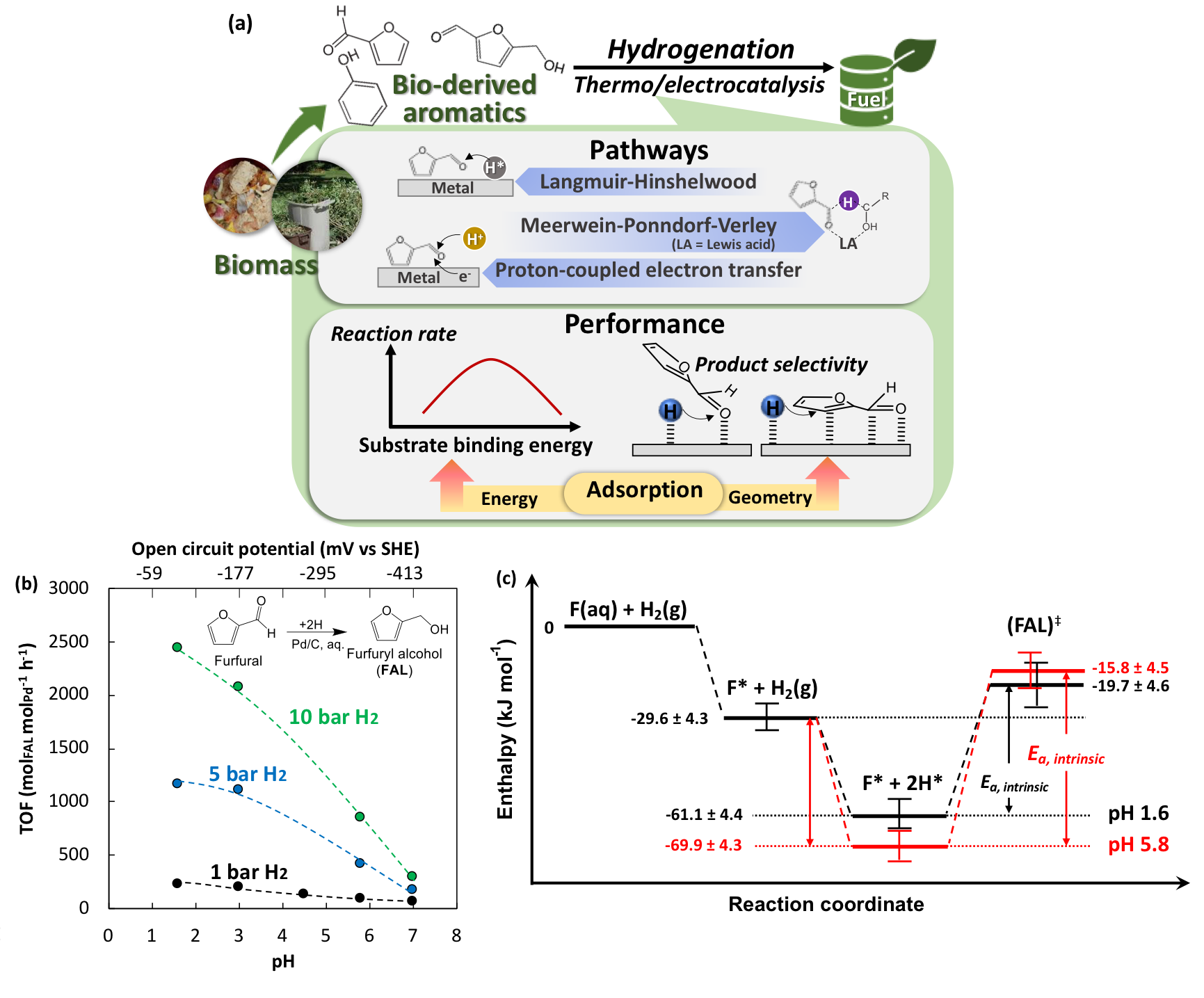
Figure 3. (a) Pathways and determinants of performance in catalytic hydrogenation of bio-derived aromatics; (b) rate of hydrogenation of furfural to furfuryl alcohol as a function of solution pH; (c) energy diagram illustrating the impact of pH on hydrogen binding energy. (Green Chem., 2021, 23, 9239-9253; Nature Comm., 2022, 13, 7154)
For more details, please contact:
Dr. Iris Yu
Email: irisyu@nus.edu.sg

