
Since the outbreak of COVID-19, there has been a global surge in demand for nasopharyngeal (NP) swabs, a key element in testing for SARS-CoV-2. Singapore is no exception. Commercially available NP swabs used in COVID-19 test kits are often out of stock due to supply chain disruptions.
To help address the global shortage, and to ensure Singapore has a sustainable supply of these swabs, two multidisciplinary research teams from NUS have developed a total of three swab designs that are comparable to the current 'gold standard' swabs.
A team led by Professor John Eu-Li Wong, NUS Senior Vice President (Health Innovation & Translation), Associate Professor Yen Ching-Chiuan, Co-Director of the Keio-NUS CUTE Center, and Professor Jerry Fuh, Director of the NUS Centre for Additive Manufacturing (AM.NUS), developed a 3D-printed swab named 'Python'. The team worked with Associate Professor David Allen and Professor Wang De Yun from NUS Yong Loo Lin School of Medicine, researchers from the NUS School of Design and Environment and NUS Faculty of Engineering, as well as clinicians from the National University Hospital (NUH), on the design, pre-clinical testing and clinical validation.
Additionally, to further increase the production of suitable swabs for Singapore and the region, another NUS team led by Professor Freddy Boey, NUS Deputy President (Innovation & Enterprise), introduced two new designs, named 'IM2' and 'IM3', that could be manufactured using injection moulding.
Nasopharyngeal swabs and screening accuracy
NP swabs are flexible sticks with a carefully designed tip section. They are inserted through the nose to the back of the nasal cavity to collect fluid samples from an individual.
NP swabs used for COVID-19 testing have a carefully designed tip section that serves to collect and retain sufficient nasopharyngeal fluids which are channelled into a holder for further testing. These are critical design factors as they could affect the accuracy of the test result when screening for SARS-CoV-2.
Python, the 3D-printed swab
To derive a viable alternative, the NUS team led by Prof Wong, Assoc Prof Yen, Assoc Prof Allen, and Prof Fuh combined their experience to design, test, and clinically validate the utility of a 3D printed NP swab for the identification of SARS-CoV-2. After considering the clinical requirements of the swab in relation to its mechanical, material and biological properties, a double helix structure was used for the swab tip, as it had excellent fluid adsorption and caused minimal discomfort to the patient.
The clinical efficacy of the swab, named Python, was compared to an industry standard swab in NUH. This was carried out in a case-controlled study of 40 patients diagnosed with COVID-19, and 10 control patients with acute respiratory illness who had tested negative for SARS-CoV-2.
The Python swab, which was developed in less than two months, demonstrated comparable accuracy and performance, with no significant difference against the standard swab. As such, the Python swab was deemed safe and acceptable for patient use, and could help mitigate strained resources in the escalating COVID-19 pandemic. A patent for the Python swab has been filed, and two local companies - Structo Pte Ltd and Eye-2-Eye Communications Pte Ltd - are mass-producing the swabs.
Assoc Prof Yen, one of the lead researchers of the project, said, "We are pleased to contribute our expertise to address the pressing need for nasopharyngeal swabs. As part of NUS' contribution towards the nation's fight against COVID-19, the design of the Python swab is free for use in Singapore."
A large-scale solution: Injection moulded swabs
To further increase the production of NP swabs, another NUS team led by Prof Boey developed an injection moulding process.
"Injection moulding as a manufacturing process is inherently faster than 3D printing as multiple units of the swabs can be produced in a given cycle. Several hundred swabs can be produced in a few minutes using the moulding process," explained Prof Boey.
His team introduced a new design that could be injection moulded, named IM2. It was tested for its mechanical strength after sterilisation, and for its efficacy in picking up viral loads. The results showed that its performance was comparable to the current commercially used swabs.
The team also redesigned the Python swab to enable it to be injection moulded by increasing the circumferential strength, and working with the manufacturer to design a mould that enables the moulding of the central hollow cavity. This design was coined IM3. Similar tests were performed to show that the design was comparable to the current commercially used swabs.
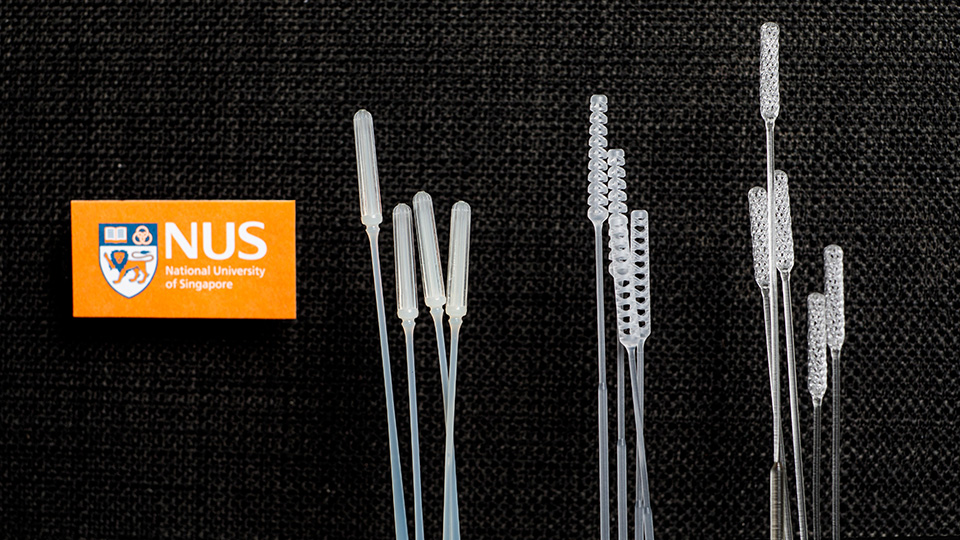
Within three months, the NUS team designed and tested these injection moulded swabs, and is carrying out clinical trials in NUH and the Singapore General Hospital. Patents have been filed for the two designs.
"The use of injection moulding ensures a secure source to supply high volumes of swabs at a low cost, to meet the needs of our community and beyond. This is crucial in our continued fight against the pandemic. The NUS IP for the injection moulded designs will also be available free for use in Singapore," shared Prof Boey, who also led the research team that designed the portable Droplet and Aerosol Reducing Tent (DART), which provides additional protection for healthcare workers.
Prof Boey and his team are now working with four companies, TNC Optics & Technologies Pte Ltd, Meiban Group Pte, Inzign Pte Ltd and Forefront Medical Pte Ltd, to mass manufacture and sterilise the injection moulded swabs.
About 40 million pieces of the IM2 and IM3 swabs are expected to be produced over the next few months, and these locally-produced swabs will be priced lower than the current commercially available imported swabs.
NUS President Professor Tan Eng Chye said, "As countries progressively emerge from lockdowns and reopen their economies, mass, repeated, testing is being widely adopted as a key public health strategy to prevent a resurgence of COVID-19 infections. This has resulted in a global shortage of commercial nasopharyngeal swabs.
We are proud that our researchers have stepped forward at this time of need. They have worked very hard, under great time pressure, to design, test and clinically validate three types of nasopharyngeal swabs within a few short months, so that these swabs can be mass manufactured locally and affordably to meet Singapore's needs."
These custom-designed NP swab designs are part of the various innovations and solutions developed by NUS researchers to tackle the COVID-19 pandemic. The University has been actively working on initiatives to fight the coronavirus since it began, with research ranging from diagnostic tests to vaccine development, as well as tapping on information and technology for solutions.
More information on NUS' efforts to address the COVID-19 pandemic can be seen here.
This article was first published on 13 Jul 2020 in NUS News.





