-
-
- Bridging Scales from Below: The Role of Heterogeneities in the Global Water and Carbon Budgets
- Increasing Occurrences of Cyanobacterial Blooms Driven by Climate Change Factors
- Carbon Capture and Utilization
- Integrated Coastal-Inland Flood Model for Climate Change
- Pathways for Sustainable and Climate-Resilient Planning of Water-Energy-Food Security Nexus
-
- Air Quality and Health: A Paradigm Shift
- Surface Water Quality and Emerging Contaminants
- Microbial detoxification of persistent organohalide pollutants (POPs)
- Nutrients Removal in Waterbodies via Sustainable Pathways
- Centre for Water Research (CWR) researchers join their forces with U of T researchers for microplastics pollution detection and control in water and wastewater
- Dealing with Hard-To-Treat Industrial Wastewater
- Valorization of Bioresources – Towards a Circular Economy
-
- Intelligent Traffic Diffusion Plan Generation, Effective Assessment and Dissemination Strategies
- Transforming Waste into Resources for Infrastructural Development
- Look-Ahead Integrated Geophysical Investigation System (IGIS) for Singapore Tunnels
- Next-Generation Airport Pavements with Full-Scale Instrumented Testing
-
- Centre for Advanced Materials and Structures (CAMS)
- Centre for Hazards Research (CHR)
- Centre for Resilient Underground Infrastructure and Engineering (CRUISE)
- Centre for Transportation Research (CTR)
- Centre for Water Research (CWR)
- Centre for Resource Circularity and Resilience (CR)2
- Centre for Offshore Research and Engineering (CORE)
- Centre for Environmental Resilience (CES)
- Safety & Health Committee
- Completed Research Projects
- Research Brief
- Achievements (in the media)
Carbon Capture and Utilization
Atmospheric carbon dioxide (CO2) concentrations continue to rise rapidly, contributing to global climate change. CO2 pollution is emitted from carbon-based fossil fuels used for transportation and electrical generation, cement manufacturing, deforestation, agriculture, and many other practices. There is a widespread consensus that the development and deployment of a broad portfolio of advanced technologies would be the most effective and sustainable approach to bring about stabilization of the atmospheric CO2 concentrations. While energy efficiency improvements and increased use of renewable energy resources are a long-term proposition of this portfolio, carbon capture, utilization and storage (CCUS) is a short to medium term technological option for mitigating anthropogenic energy-related CO2 emissions. Of the various strategies and numerous technologies that are currently being explored to capture CO2 from fossil-fuelled power plants and other large industrial sources, post-combustion capture using porous adsorbents constitutes a promising solution because of its simplicity and cost effectiveness. As a result, many different types of solid adsorbents have been explored by the scientific community among which graphene-based porous adsorbents are of current interest. Because of their porous structure together with the exceptional intrinsic properties of monolayer graphene, nanoporous graphene materials have high specific surface areas and large pore volumes, which ensure a high and rapid adsorption of CO2 from flue gas. They also have adequate robustness and mechanical strength to remain stable upon repeated exposure to hot and humid conditions and are therefore extremely suitable to be applied on an industrial scale for reducing CO2 emissions.
A major challenge is the large-scale production of graphene at low cost because of high consumption of energy. Additionally, the yield and quality of graphene obtained from conventional synthetic methods are less than satisfactory, which restrict the commercial production of graphene products. Furthermore, traditional two-dimensional (2D) graphene products tend to undergo agglomeration, when used in practical applications, and its effective surface area gets reduced. Consequently, the synthesis of three-dimensional (3D) graphene is widely explored. Biowaste-derived graphene (BG) possesses a great potential to overcome this challenge because its production is cost-effective, quality-controllable, eco-friendly, and scalable. The lignin component of lignocellulosic biowastes in particular possesses great potential as an ideal precursor for synthesis of biowaste-derived 3D graphene under mild conditions.
We have recently developed a novel synthetic approach involving modified hydrothermal carbonization (HTC) and pyrolysis graphitization (PG) to produce three-dimensional (3D) biowaste-derived graphene (3D BG) for practical CO2 capture applications. Notably, 3D biowaste-derived graphene macrostructure consists of flat and ordered graphene sheets, originated from the self-polymerization process of aromatic segments (lignin units and aromatic conjugation) and synchronous graphitization during modified HTC and PG processes as shown in Figure 1. Based on elemental and structural characterization results, 3D biowaste-derived graphene macrostructure possesses significantly increased sp2 hybridized carbon atoms and ordered graphene structures with less defect and stacking.
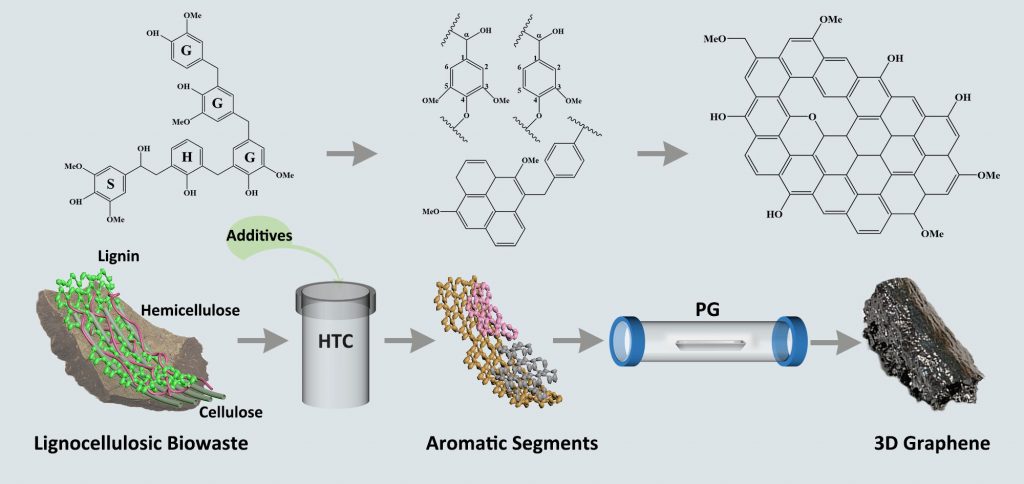
Figure 1: Production of 3D graphene by the self-polymerization and graphitization processes of lignocellulosic biowaste
3D BG exhibits a significantly improved specific surface area and pore volume compared to its pristine counterparts, and can be effectively used in post-combustion CO2 adsorption systems because of its intrinsic property together with good gravimetric storage capacity, rapid removal capability, and superior cycling stability. The adsorption performance of 3D BG towards CO2 is shown in Figure 2. The rapid adsorption of CO2 to replace adsorbed N2 (commonly found in flue gas) at 35o C indicates the excellent adsorption capability of 3D BG and its high selectivity for the CO2 capture, suggesting the possibility of recovering highly pure CO2 for long-term sequestration and/or utilization for downstream applications.
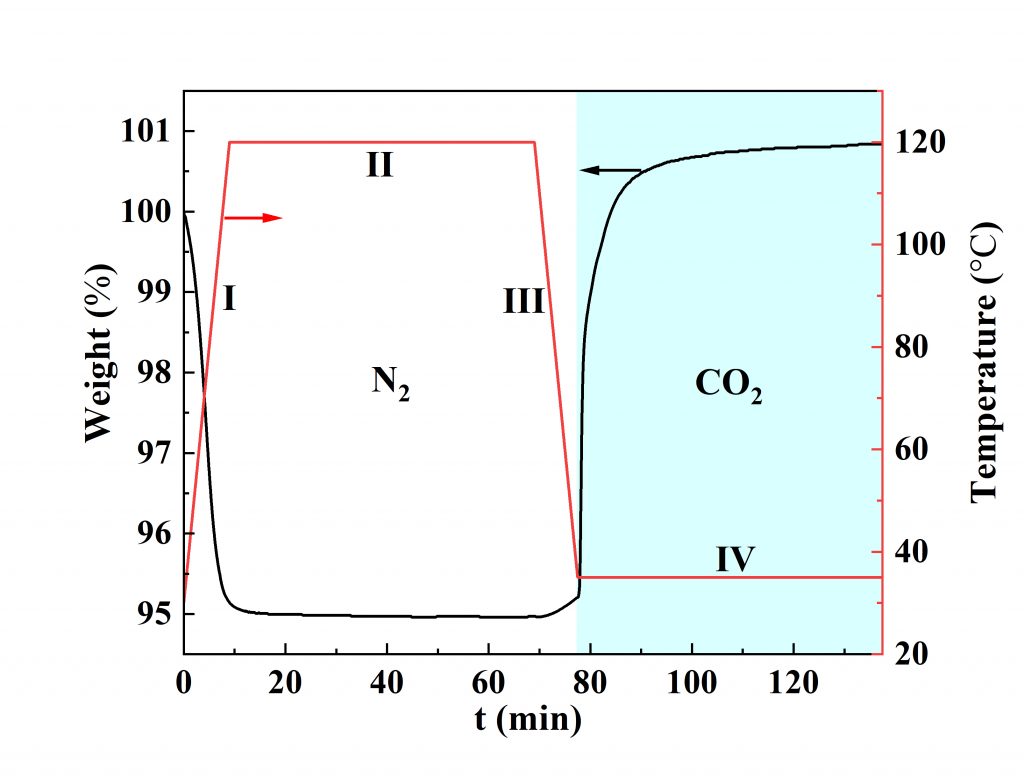 Figure 2: Selective adsorption of CO2 over N2 by 3D BG
Figure 2: Selective adsorption of CO2 over N2 by 3D BG
The novel synthesis of 3D biowaste-derived graphene and the application of this material for the CO2 capture provide new insights into developing affordable, sustainable, and advanced materials of industrial importance as well as practical relevance, contributing to circular economy.
For more details, please contact:
Prof Rajasekhar Balasubramanian
Email: ceerbala@nus.edu.sg

