Important Notice
1) All new applications for ethics review by the CDE Ethics Review Committee (CDE ERC) must be submitted through the DERC Review and Approval Management Application (DREAM App).
2) With effect from 01 March 2026, it will be mandatory for all NUS students and NUS faculty researchers (i.e., all NUS PIs and NUS Co-Is) to complete the Collaborative Institutional Training Initiative (CITI) Program's “Social & Behavioural Research - Basic/Refresher” course and upload their course completion certificates via the DREAM App.
CDE Ethics Review Committee
Ethics Review of Human Research
At the College of Design and Engineering (CDE), all student and faculty research involving human subjects as research participants and/or human tissues/cells/data must be subject to ethics review by either the NUS Institutional Review Board (NUS-IRB) or the CDE Ethics Review Committee (CDE ERC).
All student and faculty research at CDE involving human research participants can be reviewed by the CDE ERC, instead of the NUS-IRB, provided that the research meets the necessary requirements e.g., involves no more than minimal risk to participants; does not fall under the Human Biomedical Research Act (HBRA); does not involve any testing of a medical device or health product as defined in the Health Products Act (HPA); etc. Please refer to the DERC section of the NUS IRB website for more information on the research that qualifies for DERC review by the CDE ERC.
Kindly note that all research which cannot be reviewed by the CDE ERC should be submitted to the NUS-IRB for review.
Overview of Ethics Review Process
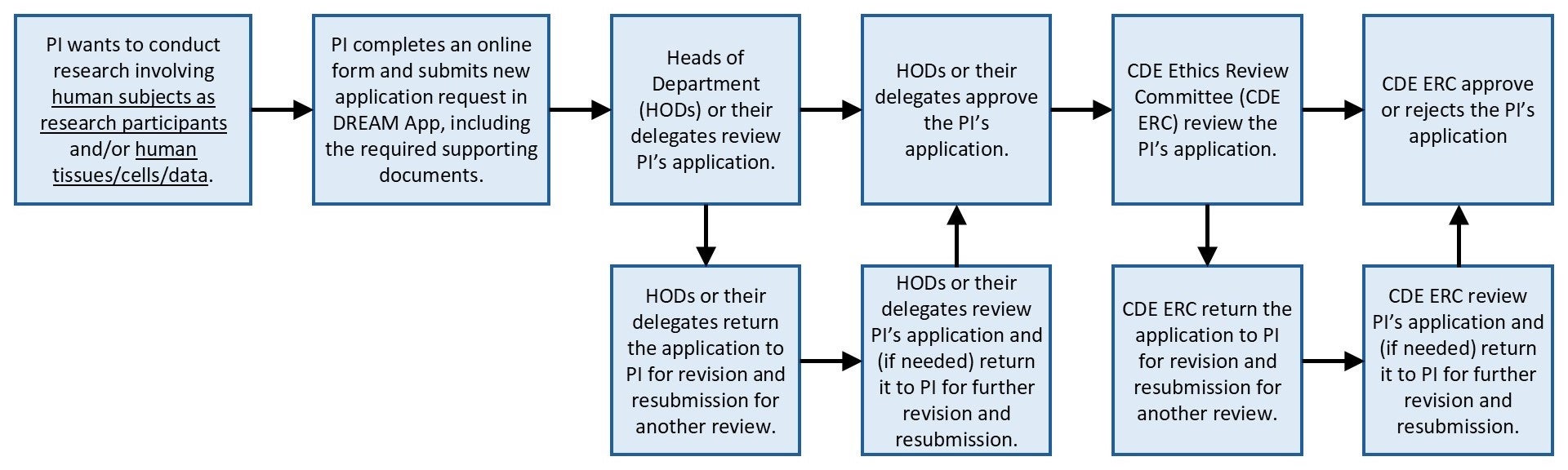
To submit the student/faculty research for ethics review by the CDE ERC, the Principal Investigator is required to complete an online application form on the DERC Review and Approval Management Application (DREAM App) and upload the required supporting documents for a complete application package. Please see the flowchart below for an overview of the ethics review process by the CDE ERC, and the following table for the required supporting documents. The Principal Investigator may start their research only after their application has been approved by the CDE ERC or the NUS-IRB.
| Required Supporting Documents | Student Research (for both undergraduate and graduate students) | Faculty Research | Additional Details |
| Data collection instruments. | Applicable. | Applicable. | Kindly upload under the “Methodology” section, at the “Study Type Supporting Documents” sub-section.
This can be survey questionnaire, interview guide, and focus group discussion guide/questions. |
| Recruitment materials. | Applicable. | Applicable. | Kindly upload under the “Recruitment” section, at the “Recruitment Supporting Documents” sub-section.
This can be recruitment advertisements, posters, and email invitations. Please refer to IRB-GUIDE-S04 Guidelines on Advertisements for Recruitment of Research Participants for more details. |
| Participant Information Sheet and Consent Form. | Applicable. | Applicable. | For Student Research: Kindly upload under the “Consent” section, at the “Participant Information Sheet & Consent Form” sub-section.
For Faculty Research: Kindly upload under the “Supplementary” section, at the “Supplementary Attachments” sub-section. Please refer to CDE ERC Guidelines on Participant Information Sheet & Consent Form (Version 2, dated 21 November 2023) for more details. |
| Collaborative Institutional Training Initiative (CITI) Program certificate for “Social & Behavioral Research - Basic/Refresher” Course. | Applicable to all PIs and Co-Is. | Applicable to all PIs and Co-Is. | Kindly upload under the “Supplementary” section, at the “Supplementary Attachments” sub-section.
With effect from 01 March 2026, it will be mandatory for all NUS students and NUS faculty researchers (i.e., all NUS PIs and NUS Co-Is) to complete the CITI Program's “Social & Behavioural Research - Basic/Refresher” course and upload their course completion certificates via the DREAM App. Please refer to NUS-IRB News Alerts #50 for more details. Please refer to Collaborative Institutional Training Initiative Programme Info for more details. |
| Supervisor’s email endorsement of the student research. | Applicable. | Not applicable. | Kindly upload under the “Supplementary” section, at the “Supplementary Attachments” sub-section.
This requirement is applicable for both undergraduate and graduate students. |
| Head of Department (HOD) Assessment Form | Only applicable if the HOD or their delegate are part of the study team. | Only applicable if the HOD or their delegate are part of the study team. | Kindly upload under the “Supplementary” section, at the “Supplementary Attachments” sub-section.
If the HOD or their delegate are part of the study team, the HOD Assessment Form should be completed by CDE Vice Dean (Research and Technology) or CDE Dean. Please refer to HOD Assessment Form for more details. |
For more details on the DREAM App and the entire ethics review process, please refer to the Applicant Guide.
For more details on the ethics review of student research, please refer to IRB-GUIDE-018 Ethics Review of Student Research Involving Human Research Participants.
For more details on the ethics review of faculty research, please refer to IRB-GUIDE-022 Ethics Review of Faculty Research that Qualify for an IRB exemption by the Departmental Ethics Review Committee.
Amendments and Extensions for Previously Approved Research:
If the research was previously approved by the CDE ERC, and subsequently requires an amendment or extension, the Principal Investigator should submit the appropriate request on the DERC Review and Approval Management Application (DREAM App). For more details, please refer to the Applicant Guide.
Likewise, the Principal Investigator may start/resume their amended research only after their request has been approved by the CDE ERC.
Information and Resources:
With effect from 01 March 2026, the NUS-IRB will implement mandatory Collaborative Institutional Training Initiative (CITI) Program certification for all NUS staff and students conducting Social, Behavioural and Educational Research (SBER). For all ethics review applications to the CDE ERC, it will be mandatory for all NUS students and NUS faculty researchers (i.e., all NUS PIs and NUS Co-Is) to complete the CITI Program's “Social & Behavioural Research - Basic/Refresher” course and upload their course completion certificates via the DREAM App, from 01 March 2026 onwards. This is to ensure a consistent and high standard of ethical oversight for all human subject research at NUS, as well as provide researchers with targeted training on SBER-specific topics. Please refer to NUS-IRB News Alert #50 for more details.
When would student projects require ethics review? If the primary aim of the student project is to impart methods or offer students hands-on experience in the research process without contributing to generalizable knowledge, it may not necessitate ethics review. However, if the aim is to generate generalizable knowledge and to submit the results to conferences or competitions, or publish them in journals or on websites, it will fall under NUS-IRB’s definition of research and hence requires ethics review. Ultimately, it is the supervisors’ responsibility to determine and decide whether the student project qualifies as an “educational exercise” involving human subjects or if it constitutes “research”. Please refer to NUS-IRB News Alert #44 and the IRB Advisory 01 for more details.
Photography and video recordings that capture the participants’ faces are classified as “personal data” under the Personal Data Protection Act. In the Participant Information Sheet (PIS), the researcher should inform the participants (i) what is the purpose of capturing their photo and video, (ii) what will the photos and videos be used for, (iii) whether their faces will be blurred or not be blurred before use, and (iv) when will photos and videos be deleted (i.e., for how long will photos and videos be stored for). In the Consent Form, the researcher should include an option for the participants to agree or disagree to photography and video recording. Please use the standard verbiage from the CDE ERC Guidelines on Participant Information Sheet & Consent Form.
Social media data/posts may fall under “human subjects research”. Please see Category 4B under the List of SBER Exemption Categories for more examples of such research. The researcher should provide more details of (i) what types of posts will be analysed, (ii) whether any posts will be reproduced (e.g., screenshots used) in their thesis, publications, etc. The researcher should also apply for a waiver of informed consent in the ethics application form if required (see Category 4B under the List of SBER Exemption Categories). Alternatively, the researcher should seek permission for the use of social media posts (if feasible).
Data collection instruments are essential components in collecting research data from the participants. In the future, if there are any changes to the survey questionnaire, the researcher should submit a modification or amendment to the CDE ERC and attach the latest version for the CDE ERC’s review, before enacting the changes. For interview or focus group questions, it may not be possible to define exactly what questions will be asked, but the researcher can make clear to participants that the initial set of questions is the starting point of the conversation. Please refer to the above section "Amendments and Extensions for Previously Approved Research" for more details on post-approval revisions.
Non-NUS students should be aged 21 and above if recruited. If they are under 21 years old, parental consent must be obtained.
Available resources from the NUS-IRB:
- NUS-IRB's Social, Behavioural and Educational Research (SBER) Review Guidelines.
- NUS-IRB's Departmental Ethics Review Committee (DERC) Guidelines and Resources.
- NUS-IRB News Alerts
- NUS-IRB Homepage.
- Applicant Guide for the DREAM App.
- HOD Guide for the DREAM App.
- DERC Guide for the DREAM App (only accessible for CDE ERC Chair and Members).
About the CDE Ethics Review Committee (CDE ERC):
The CDE ERC is currently chaired by Associate Professor KHOO Eng Tat, and the members include:
- [BME] Assistant Professor CHEOW Lih Feng
- [BME] Assistant Professor Andrew William HOLLE
- [CEE] Assistant Professor Prateek BANSAL
- [CEE] Associate Professor Olivier Patrick LEFEBVRE
- [CEE] Associate Professor LIU Yang
- [CEE] Assistant Professor Iris YU
- [ChBE] Associate Professor LIM Wee Chuan, Eldin
- [ChBE] Assistant Professor LOH Tzu Yang
- [DBE] Dr LIN Alexander
- [DBE] Associate Professor TAN Meng Hor, Freddie
- [DBE] Associate Professor TAY En Rong, Stephen
- [DID] Assistant Professor CHO Janghee
- [DID] Associate Professor LEE Jung Joo
- [DID] Assistant Professor Gabriel Elijah LIPKOWITZ
- [DID] Associate Professor YEN Ching-Chiuan
- [DOA] Associate Professor LAU Siu Kit, Eddie
- [DOA] Assistant Professor TANG Dorothy
- [DOA] Associate Professor TRIVIC Zdravko
- [ECE] Professor GE Shuzhi Sam
- [EDIC] Associate Professor Mark Philip DE LESSIO
- [EDIC] Dr LEE Sang Won, Kate
- [EDIC] Dr TANG Kok Zuea
- [ISEM] Dr GOH Tian
- [ISEM] Dr KUO Vincent
- [ME] Associate Professor CHUI Chee Kong
- [ME] Associate Professor THIAN Eng San
Contact Information:
If you have any questions, or require an independent opinion on any research conducted by CDE student or faculty member, please contact a staff member of the CDE Ethics Review Committee (CDE ERC) at cdebox5@nus.edu.sg.


